Abstract
Introduction: Generic formulations of imatinib were approved and commercially available in the United States starting in 2016, introducing vast cost savings to the standard treatment of chronic myeloid leukemia (CML). While bioequivalence studies of generic formulations are required for Food and Drug Administration approval, the safety of generic drug supply chains have come into question. There is limited real-world data comparing the effectiveness and safety profiles of generic formulations to the original. This study aimed to evaluate the effectiveness and safety of generic imatinib compared to the branded product.
Methods: This retrospective study included patients treated at UNC Medical Centers who were diagnosed with CML and treated with imatinib at any time during their course of treatment. Data was retrieved from the institution's electronic health record and collected over the first 6 months of imatinib treatment to include both safety and effectiveness outcomes. The primary endpoint was to compare generic versus branded product effectiveness, as defined by the European LeukemiaNet (ELN) guidelines (achieving BCR-ABL/ABL ratio of <10% and <1% at 3 and 6 months, respectively). The secondary endpoints included comparisons of generic vs branded product safety, measured via patient adverse drug events (ADEs), all-cause hospitalizations, and early treatment discontinuation. Patients were excluded from primary endpoint evaluation and only included for safety endpoint analysis if they were not treated with imatinib first-line and if duration of imatinib treatment was less than 6 months.
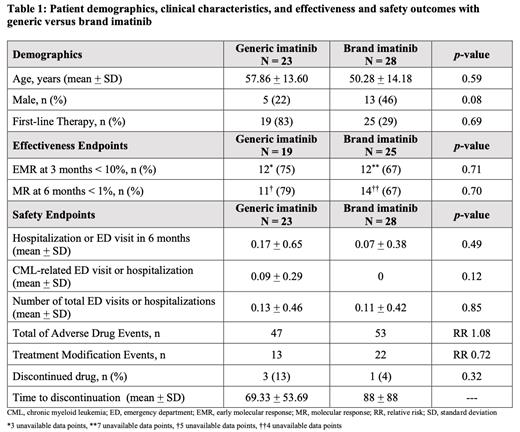
Results: Fifty-one CML patients met criteria with no significant differences in age or gender between the generic (n = 23) and brand (n = 28) imatinib groups (Table 1). First-line therapy was composed of 83% of patients on generic imatinib and 29% of patients on brand imatinib. Of those receiving first-line imatinib therapy, there was no difference in molecular responses at 3 and 6 months between generic and brand imatinib (p = 0.71). Brand imatinib was associated with numerically lower CML-related emergency department visits and hospitalizations when compared to generic imatinib, although this difference was not statistically significant (p = 0.12). Rates of discontinuation were numerically lower for brand imatinib although overall time to discontinuation was shorter for generic imatinib (Table 1).
Conclusions: This study demonstrates real-world treatment effectiveness and safety of generic and brand imatinib in clinical practice. Generic imatinib appears to be associated with higher rates of CML-related ED visits and hospitalizations although sample size was small and statistical significance was not reached. Further analyses of comparisons and continuation of data collection will provide a more robust assessment to compare the effectiveness and safety of generic and brand imatinib in the real-world setting.
Muluneh: Novartis: Other: Spouse works for Novartis.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal